More Information
Submitted: October 13, 2022 | Approved: October 25, 2022 | Published: October 26, 2022
How to cite this article: Zhukov KV, Gasparyan BA, Vetcher AA, Shishonin AY. Left ventricular hypertrophy linked with arterial hypertension through centralized aerobic-anaerobic energy balance compensation theory. Ann Clin Hypertens. 2022; 6: 012-014.
DOI: 10.29328/journal.ach.1001030
Copyright License: © 2022 Zhukov KV, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Left ventricular hypertrophy; Arterial hypertension; Centralized aerobic-anaerobic energy balance compensation theory
Left ventricular hypertrophy linked with arterial hypertension through centralized aerobic-anaerobic energy balance compensation theory
Kirill V Zhukov1, Bagrat A Gasparyan1, Alexandre A Vetcher1,2* and Alexander Y Shishonin1
and Alexander Y Shishonin1
1Complementary and Integrative Health Clinic of Dr. Shishonin, 5 Yasnogorskaya Str, Moscow, 117588, Russian Federation
2Peoples’ Friendship University of Russia (RUDN), 6 Miklukho-Maklaya St, 117198 Moscow, Russia
*Address for Correspondence: Alexandre A Vetcher, Complementary and Integrative Health Clinic of Dr. Shishonin, 5 Yasnogorskaya Str, Moscow, 117588, Russian Federation, Email: [email protected]
We demonstrated intrinsic connections between left ventricular hypertrophy (LVH) and arterial hypertension (AHT) through the recently announced centralized aerobic-anaerobic energy balance compensation (CAAEBC) theory. CAAEBC has already demonstrated achievements in the treatment of AHT, diabetes myelitis (DM), and osteochondrosis. Such demonstration lifts the necessity to check the applicability of this theory to other non-communicable diseases (NCDs) and develop the proper way to model the main idea of CAAEBC.
LVH is a condition in which an increase in left ventricular mass occurs secondary to an increase in wall thickness, an increase in left ventricular cavity enlargement, or both [1]. Most commonly, the left ventricular wall thickening occurs in response to pressure overload, and chamber dilatation occurs in response to volume overload. No doubt, it is associated with AHT [2-4].
For a while, the statistical correlation of AHT with brachiocephalic arterial blood [5-8] was mentioned as required theoretical consideration. And so far, just one pure theoretical analysis, where AHT was considered as a result of the obstructions of blood access to the brain, had been reported [9].
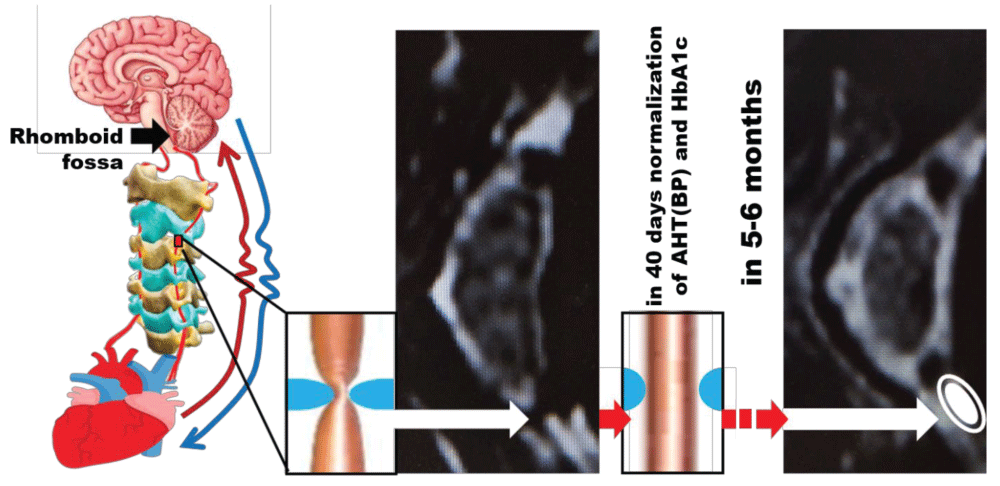
Recently we explained through the development of CAAEBC theory why the access to arterial blood flow to the rhomboid fossa is so critical to the body’s internal conditions regulation [10]. The visual explanation is exhibited in Figure 1.
Figure 1: Schematic of observed of consequences of blood access to the rhomboid fossa restoration.
So far, we have demonstrated, that the proposed approach helps the body regain control of arterial blood pressure (BP) [11], HbA1c [12] and vertebral cartilage [13].
Should we check if LVH is on this list? To answer this question, first of all, it is necessary to check if LVH, caused by AHT, can be recovered. Indeed, the successful recovery was reported over two decades ago, but it becomes statistically noticeable only after half of the year of therapy [14]. To reach a plateau the observation should last at least three years. Therefore, it is worth trying. In this case, the initial scheme, where the first and last measurements of desired parameters we take approximately six weeks apart [10-13] should be corrected according to the time course of partial normalization of LVH in case of AHT recovery [14].
LVH is, as mentioned in the Introduction, an abnormal increase in left ventricular mass. It is associated with coronary events, stroke, heart failure, peripheral arterial disease, and cardiovascular mortality in patients with AHT [4,15]. LVH is usually detected by electrocardiography, echocardiography, and magnetic resonance imaging. According to the American Society of Echocardiography and the European Association of Cardiovascular Imaging, LVH is defined as an increased left ventricular mass index (LVMI) greater than 95 g/m in women and increased LVMI to greater than 115 g/m in men [1]. It is divided into two groups – concentric and eccentric. A concentric LVH is an increased left ventricular mass index (LVMI) with a relative wall thickness ≥ 0.45, while eccentric LVH -< 0.45 [4,15]. Increasing age in patients with AHT as well as diabetes mellitus in patients with AHT is associated with concentric LVH, whereas obesity, which is a volume overload state, and coronary artery disease in patients with AHT - with eccentric [16].
CAAEBC theory suggests that the restoration of the above-mentioned access with the subsequent strengthening of the cervical muscular corset will eventually lead to the normalization of the majority of internal body functions and, therefore, corresponding parameters. Therefore, we need to set the acquisition of LWMI before the therapy and six months after its completion.
It would be also convenient to step from medical record analysis to the experiment. The experimental data collection should be done on the appropriate animal model(s), the choice of which in contemporary conditions is associated with a wide variety of issues [17-25].
We demonstrate that the next steps to prove CAAEBC theory applicability to LVH should be
- Analysis of LVMI data taken before and six months after therapy in the Clinic
- Experiments on appropriate animal model.
Author contributions
Conceptualization, A.Y.S., A.A.V., B.A.G., A.Y.S.; writing—original draft preparation, A.Y.S., A.A.V.; writing—review and editing, A.Y.S., A.A.V.; visualization, A.A.V.; supervision, A.Y.S., and A.A.V.; project administration K.V.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This paper has been supported by the RUDN University Strategic Academic Leadership Program (recipient A.A.V.).
The authors wish to thank BS V.D. Bystrykh for her assistance with the editing of the submission’s final version. Alexandre Vetcher expresses acknowledgments to the RUDN University Strategic Academic Leadership Program for the obtained support.
- Bornstein AB, Rao SS, Marwaha K. Left Ventricular Hypertrophy. Treasure Island (FL): StatPearls Publishing; 2022.
- Cuspidi C, Sala C, Negri F, Mancia G, Morganti A; Italian Society of Hypertension. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012 Jun;26(6):343-9. doi: 10.1038/jhh.2011.104. Epub 2011 Nov 24. PMID: 22113443.
- Kahan T, Bergfeldt L. Left ventricular hypertrophy in hypertension: its arrhythmogenic potential. Heart. 2005 Feb;91(2):250-6. doi: 10.1136/hrt.2004.042473. PMID: 15657259; PMCID: PMC1768675.
- Aronow WS. Hypertension and left ventricular hypertrophy. Ann Transl Med. 2017;5(15):310. doi: 10.21037/atm.2017.06.14.
- Whelton PK, Einhorn PT, Muntner P, Appel LJ, Cushman WC, Diez Roux AV, Ferdinand KC, Rahman M, Taylor HA, Ard J, Arnett DK, Carter BL, Davis BR, Freedman BI, Cooper LA, Cooper R, Desvigne-Nickens P, Gavini N, Go AS, Hyman DJ, Kimmel PL, Margolis KL, Miller ER 3rd, Mills KT, Mensah GA, Navar AM, Ogedegbe G, Rakotz MK, Thomas G, Tobin JN, Wright JT, Yoon SS, Cutler JA; National Heart, Lung, and Blood Institute Working Group on Research Needs to Improve Hypertension Treatment and Control in African Americans. Research Needs to Improve Hypertension Treatment and Control in African Americans. Hypertension. 2016 Nov;68(5):1066-1072. doi: 10.1161/HYPERTENSIONAHA.116.07905. Epub 2016 Sep 12. PMID: 27620388; PMCID: PMC5063700.
- de Simone G, Mancusi C, Esposito R, De Luca N, Galderisi M. Echocardiography in Arterial Hypertension. High Blood Press Cardiovasc Prev. 2018 Jun;25(2):159-166. doi: 10.1007/s40292-018-0259-y. Epub 2018 May 2. PMID: 29721914.
- Finocchi C, Sassos D. Headache and arterial hypertension. Neurol Sci. 2017 May;38(Suppl 1):67-72. doi: 10.1007/s10072-017-2893-x. PMID: 28527058.
- Wermelt JA, Schunkert H. Management of arterial hypertension. Herz 2017;42(5): 515–26.
- Ermoshkin VI. Hypothesis of causeless hypertension. (in Russian) http://www. medlinks.ru/, 2011.
- Vetcher AA, Zhukov KV, Gasparuan BA, Shishonin AY. The cerebellum role in arterial hypertension. Medical Hypotheses. 2022;162:10835 doi:10.1016/j.mehy.2022.110835
- Vetcher AA, Zhukov KV, Gasparuan BA, Shishonin AY. The cervical blood flow parameters with the best correlation from arterial blood pressure in hypertension cases. International Journal of Recent Scientific Research. 2021; 09 (A):42957-42958 doi:10.24327/ijrsr.2021.1209.6184
- Vetcher AA, Zhukov KV, Gasparuan BA, Shishonin AY. Restoration of HbA1c level for pre-diabetic patients through the restoration of arterial blood flow access to rhomboid fossa. Diabetology. 2022; 3: 470–476. Doi: 10.3390/diabetology3030035
- Zhukov KV, Vetcher AA, Gasparuan BA, Shishonin AY. Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis. Polymers (Basel). 2021 Dec 3;13(23):4248. doi: 10.3390/polym13234248. PMID: 34883749; PMCID: PMC8659970.
- Franz IW, Tönnesmann U, Müller JF. Time course of complete normalization of left ventricular hypertrophy during long-term antihypertensive therapy with angiotensin converting enzyme inhibitors. Am J Hypertens. 1998 Jun;11(6 Pt 1):631-9. doi: 10.1016/s0895-7061(98)00024-7. PMID: 9657621.
- Aronow WS, Ahn C, Kronzon I, Koenigsberg M. Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am J Cardiol. 1991 Feb 1;67(4):295-9. doi: 10.1016/0002-9149(91)90562-y. PMID: 1825011.
- Drazner MH. The progression of hypertensive heart disease. Circulation. 2011; 123:327-334. Doi: 10.1161/CIRCULATIONAHA.108.845792
- Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007 May;13(5):561-6. doi: 10.1038/nm1595. PMID: 17479102; PMCID: PMC3245971.
- Geissler SA, Schmidt CE, Schallert T. Rodent Models and Behavioral Outcomes of Cervical Spinal Cord Injury. J Spine. 2013 Jul 27;Suppl 4:001. doi: 10.4172/2165-7939.S4-001. PMID: 25309824; PMCID: PMC4191831.
- Simon FH, Erhart P, Vcelar B, Scheuerle A, Schelzig H, Oberhuber A. Erythropoietin preconditioning improves clinical and histologic outcome in an acute spinal cord ischemia and reperfusion rabbit model. J Vasc Surg. 2016 Dec;64(6):1797-1804. doi: 10.1016/j.jvs.2015.10.011. Epub 2015 Nov 21. PMID: 26610640.
- Uezu T, Koja K, Kuniyoshi Y, Miyagi K, Shimoji M, Arakaki K, Yamashiro S, Mabuni K, Senaha S. Blood distribution to the anterior spinal artery from each segment of intercostal and lumbar arteries. J Cardiovasc Surg (Torino). 2003 Oct;44(5):637-45. PMID: 14735053.
- Singh VK, Thrall KD, Hauer-Jensen M. Minipigs as models in drug discovery. Expert Opin Drug Discov. 2016 Dec;11(12):1131-1134. doi: 10.1080/17460441.2016.1223039. Epub 2016 Aug 22. Erratum in: Expert Opin Drug Discov. 2017 Jul;12 (7):755. PMID: 27546211.
- Maršala M. Spinal cord blood flow and metabolism in transient spinal ischemia. In: E Stålberg, HS Sharma, Y Olsson, editors. Spinal cord monitoring. New York: Springer. 1998; 5-25.
- Pais D, Casal D, Arantes M, Casimiro M, O'Neill JG. Spinal cord arteries in Canis familiaris and their variations: implications in experimental procedures. Braz J Morphol Sci. 2007; 24: 224– 228.
- DeGirolami U, Zivin JA. Neuropathology of experimental spinal cord ischemia in the rabbit. J Neuropathol Exp Neurol. 1982 Mar;41(2):129-49. doi: 10.1097/00005072-198203000-00004. PMID: 7062084.
- National Research Council (US) Committee on Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research. Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research. Washington (DC): National Academies Press (US); 2009. 2, Use of Dogs and Cats in Research: Public Perception and Evolution of Laws and Guidelines. 1-9.